On September 18, 2025, a research team led by Professor Renjie Chai from the School of Life Science and Technology at Southeast University, in collaboration with multiple institutions, published a study in the internationally renowned journal Nature Communications titled “Optimized in vivo base editing restores auditory function in a DFNA15 mouse model”. The study focused on DFNA15, a form of dominant hereditary deafness caused by a nonsense mutation in the POU4F3 gene. The researchers developed a precise and efficient adenine base editor (ABE) and, using a dual adeno-associated virus (dual-AAV) delivery system, successfully corrected the pathogenic mutation in a deaf mouse model. This intervention led to significant restoration of auditory function, providing critical experimental evidence and a promising strategy for gene therapy in hereditary hearing loss.

Hearing loss is a major global health concern, affecting approximately 430 million people. More than half of all cases of sensorineural hearing loss are attributed to genetic factors, often resulting from dysfunction of inner ear hair cells. Conventional interventions, such as hearing aids and cochlear implants, provide partial benefit but are limited by residual auditory function and do not address the underlying genetic cause. In contrast, gene editing technologies capable of directly repairing pathogenic mutations offer promising therapeutic opportunities.
DFNA15, an autosomal dominant non-syndromic hearing loss caused by mutations in the POU4F3 gene, exhibits clinical heterogeneity depending on the mutation. The c.337C>T nonsense mutation in Chinese populations introduces a premature stop codon, producing a truncated, non-functional protein that leads to progressive bilateral hearing loss. To date, no effective treatments are available for this condition.
To explore gene therapy strategies for DFNA15, the research team first established a Pou4f3WT/Q113* mouse model that faithfully recapitulates the progressive hearing loss phenotype observed in patients. They constructed a versatile adenine base editor (ABE) toolbox by combining PAM-flexible, highly active Cas9 nickases with optimized TadA deaminases. Through in vitro screening, the SchABE8e-sgRNA3 combination was identified as the most efficient and precise editor for the target locus.
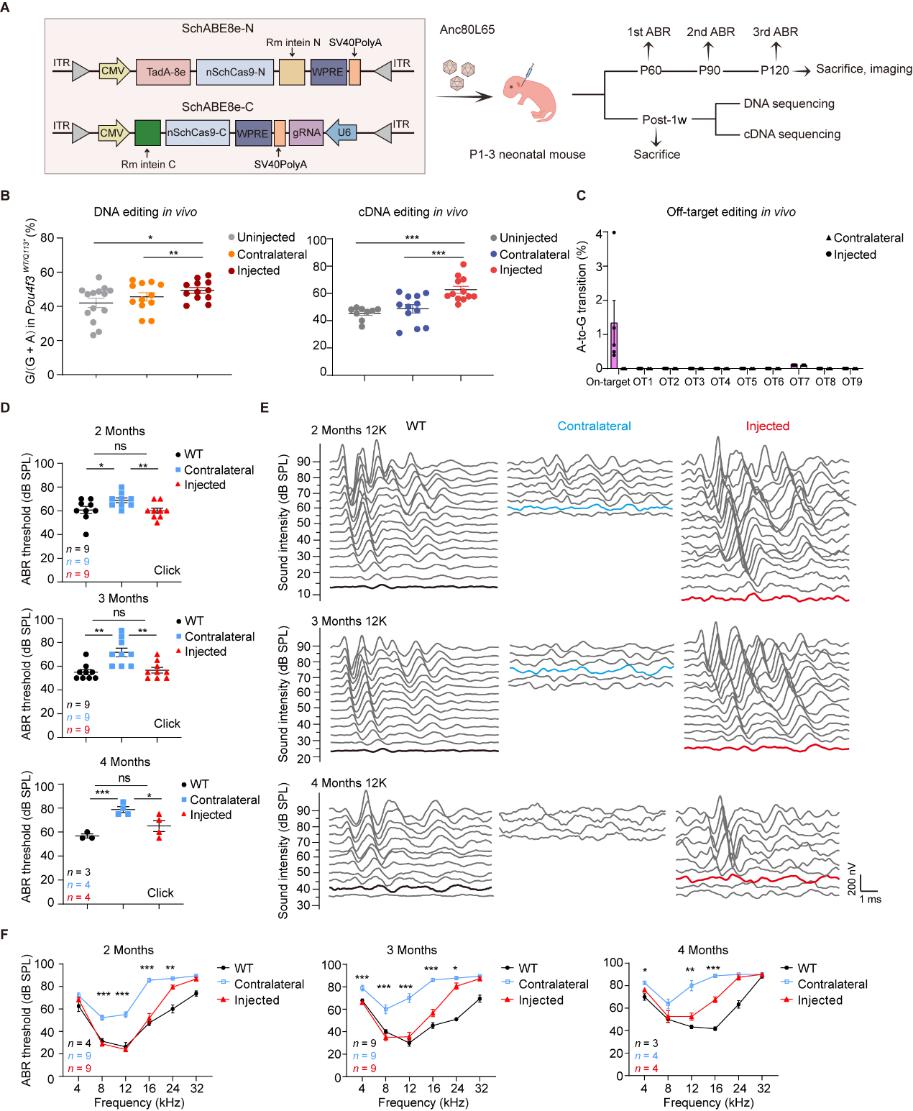
Figure | Evaluation of the therapeutic efficacy of dual-AAV-delivered SchABE8e-sgRNA3 in Pou4f3WT/Q113*mice
For validation, a dual-AAV delivery system was used to deliver SchABE8e-sgRNA3 into the inner ear. This strategy efficiently corrected the pathogenic mutation and restored both expression and nuclear localization of full-length POU4F3 protein. Audiological assessments confirmed that treated mice recovered near wild-type hearing, with therapeutic benefits sustained for at least four months. Furthermore, whole-genome sequencing (WGS) and behavioral analyses demonstrated a favorable biosafety profile, with no detectable off-target effects or toxicity in the inner ear or nervous system.
In conclusion, this study establishes a highly efficient and safe in vivo base editing platform and, for the first time, achieves long-term restoration of auditory function in an animal model of dominant hereditary deafness by directly repairing the pathogenic gene. These findings provide essential experimental evidence for the clinical translation of DFNA15 therapies and offer new insights and therapeutic options for the development of treatment strategies for other genetic diseases.
Professor Renjie Chai from the School of Life Science and Technology at Southeast University; Associate Professor Jieyu Qi from Beijing Institute of Technology; Associate Researcher Fangzhi Tan from Zhongda Hospital, Southeast University; and Researcher Hongbo Yang from Fudan University served as co-corresponding authors of this paper. The co-first authors are Ph.D. candidates Man Wang and Ziyu Zhang, Master’s candidate Xiaohan Wang, and postdoctoral fellow Liyan Zhang, all from the School of Life Science and Technology at Southeast University.
Original article link:https://www.nature.com/articles/s41467-025-63613-w
